Jan. 14, 2025
Coagulant Therapeutics Announces Publication in Blood Advances Describing APC-Targeted Antibodies for Therapeutic Modulation of Coagulopathy and Inflammation
The Company sponsored study at The Scripps Research Institute, explores APC-targeted antibodies that selectively modulate anticoagulant and anti-inflammatory activities, offering potential therapeutic solutions for conditions like traumatic bleeding, hemophilia, sepsis, and ischemia.
BERKELEY, Calif., Jan. 14, 2025- Coagulant Therapeutics Corporation, a privately held company (Company) focused on the design, development and commercialization of therapeutics related to the coagulation cascade, announced the online publication of a manuscript titled "Divergent modulation of activated protein C pleiotropic functions by antibodies that differ by a single amino acid" in the American Society of Hematology journal Blood Advances.
The Company collaborated with Laurent Mosnier, PhD, Professor, Department of Translational Medicine at The Scripps Research Institute, to investigate a series of antibodies targeting activated protein C (APC), a protease with both anticoagulant and anti-inflammatory properties. By employing directed evolution techniques to a parental antibody, TPP-26870, an antibody that binds APC at a non-active site, Coagulant developed single amino acid variants of TPP-26870. These variants resulted in selective modulation of APC's diverse biological activities, including its anticoagulant and anti-inflammatory effects. These findings open the door to explore more tailored and effective APC-based therapeutic interventions.
“Seeing how single mutations in the antibody selectively enhanced some activities of APC while inhibiting others is quite astonishing. It really shows the potential how mutations can help feature the most beneficial collection of APC activities” added Dr. Mosnier.
One key finding from the study is the identification of a specific antibody variant, the N57W VL substitution, which exhibited improved efficacy in prolonging APC’s in vitro plasma half-life, enhancing anticoagulant activity, and promoting histone cleavage. This unique collection of properties of N57W VL variant may be particularly effective for treating trauma-induced coagulopathy (TIC).
“This is the second proprietary APC library generated by Coagulant that has delivered highly differentiated antibodies with exciting and unique therapeutic features. We look forward to exploring their potential as treatments for an array of diseases of unmet medical need including trauma, hemophilia, sepsis and ischemia” said Terry Hermiston, PhD, Chief Executive Officer of Coagulant Therapeutics.
This publication furthers Coagulants leadership position in developing APC-based treatments, complementing the already published work on an independent nanobody derived antibody library (Sim DS et al. Blood Adv 2023, 7:3036). Together with work on Coagulant’s lead molecule, CT-001, an engineered next generation FVIIa under development to treat severe postpartum hemorrhage (PPH), Coagulant has now assembled a pipeline of molecules for the treatment of acute bleeding (PPH, Trauma, Traumatic Brain Injury, Intracranial Hemorrhage), the leading cause of death in those aged 1-46 for which there are currently no approved pharmaceutical therapies.
About APC Antibody Programs
APC is a pleiotropic enzyme that has been identified as a target in the treatment of a variety of disease indications including Trauma, Hemophilia, Ischemia and Sepsis. Coagulant Therapeutics Corporation has initiated research programs into the identification of APC-directed therapeutic antibodies, leveraging the unique features of llama antibodies (nanobodies) to derive novel nanobodies to the non-active sites of the protein. The nanobody library has an array of different antibodies to differing epitopes and, importantly, generated a lead which is being studied for treatment of trauma and hemophilia. In parallel, Coagulant Therapeutics Corporation initiated a program of directed evolution to a known APC antibody that also targeted the non-active sites of APC to further enrich the diversity of the toolbox available to find highly differentiated therapeutic molecules to APC and associated disease indications.
About Coagulant Therapeutics Corporation
Coagulant Therapeutics is a privately held company focused on the design, development and commercialization of therapeutics targeted to the coagulation cascade and its adjacencies. The Company’s lead product candidate, CT-001, is a next-generation Factor VIIa designed for enhanced efficacy and safety in the setting of acute bleeding. Coagulant is also developing additional therapeutics targeted to APC for the treatment of acute bleeding and other coagulation cascade-related diseases. Founded in 2019, the company is based in Berkeley, California.
Jan. 13, 2025
Coagulant Therapeutics to Present at Biotech Showcase 2025™
BERKELEY, Calif., Jan. 13-15, 2024
Coagulant Therapeutics today announced that Terry Hermiston, Ph.D., CEO and Founder, will be presenting Coagulant Therapeutics at the Biotech Showcase™ 2025 conference.
Coagulant Therapeutics, leaders in the development of treatments for acute bleeding, is developing a product candidate licensed from Bayer AG for the treatment of acute bleeding, CT-001, for severe postpartum hemorrhage (sPPH). In addition, Coagulant has generated a pipeline of additional clinical leads from its proprietary nanobody library to activated protein C (APC) to treat acute bleeding and sepsis.
“This is an exciting time for Coagulant as we work with current and future investors to drive forward our therapies for acute bleeding, led by our engineered FVIIa molecule, CT-001 said Dr. Terry Hermiston, CEO and Founder of Coagulant.
Biotech Showcase, produced by Demy-Colton and EBD Group, is an investor conference focused on driving advances in therapeutic development by providing a sophisticated networking platform for executives and investors that fosters investment and partnership opportunities. The conference takes place each year during the course of one of the industry's largest gatherings and busiest weeks.
About Coagulant Therapeutics Corporation
Coagulant Therapeutics is a privately held company focused on the design, development and commercialization of therapeutics targeted to the coagulation cascade and its adjacencies. The Company’s lead product candidate, CT-001, is a next-generation Factor VIIa designed for enhanced efficacy and safety in the setting of acute bleeding. Coagulant is also developing additional therapeutics targeted to APC for the treatment of acute bleeding and other coagulation cascade-related diseases. Founded in 2019, the company is based in Berkeley, California.
Apr. 8, 2024
Coagulant Therapeutics Announces Upcoming Oral Presentation at the 2024 International Society for Thrombosis and Hemostasis (ISTH) Congress
BERKELEY, Calif., Apr. 8, 2024
Coagulant Therapeutics Corporation, a privately held company focused on the design, development and commercialization of therapeutics targeted to acute bleeding and its adjacencies, today announced the abstract acceptance for presentation at the 32nd International Society for Thrombosis and Hemostasis (ISTH) Congress, taking place in Bangkok, Thailand at the Queen Sirikit National Convention Center, and virtually June 22-26, 2024. Abstract OC 76.1 is an oral presentation titled “Directed Evolution of an Antibody Specific for a Non-Active Site of Activated Protein C (APC) – Identification of Positions Within Each CDR for Selective Modulation of APC Pleiotropic Activities”.
ORAL PRESENTATION SESSION DETAILS
Abstract Title: Directed Evolution of An Antibody Specific for a Non-Active Site of Activated Protein C (APC) - Identification of Positions Within Each CDR for Selective Modulation of APC Pleiotropic Activities
Abstract Presentation Number: OC 76.1
Session Title: Functional regulation of natural anticoagulants
Session Date and Time: Wednesday June 26, 2024 at 10:15 - 11:30 ICT
Presentation Time: 10:15 - 10:30 ICT, 12-minute presentation + 3-minute Q&A
About APC Antibody Programs
APC is a pleiotropic enzyme that has been identified as a target in the treatment of a variety of disease indications including Trauma, Hemophilia, Ischemia and Sepsis. Coagulant Therapeutics Corporation has initiated research programs into the identification of APC-directed therapeutic antibodies, leveraging the unique features of llama antibodies (nanobodies) to derive novel nanobodies to the non-active sites of the protein. The nanobody library has an array of different antibodies to differing epitopes and, importantly, generated a lead which is being studied for treatment of trauma and hemophilia. In parallel, Coagulant Therapeutics Corporation initiated a program of directed evolution to a known APC antibody that also targeted the non-active sites of APC to further enrich the diversity of the toolbox available to find highly differentiated therapeutic molecules to APC and associated disease indications.
About Coagulant Therapeutics Corporation
Coagulant Therapeutics is a privately held company focused on the design, development and commercialization of therapeutics targeted to the coagulation cascade and its adjacencies. The Company’s lead product candidate, CT-001, is a next-generation Factor VIIa designed for enhanced efficacy and safety in the setting of acute bleeding. Coagulant is also developing additional therapeutics targeted to APC for the treatment of acute bleeding and other coagulation cascade-related diseases. Founded in 2019, the company is based in Berkeley, California.
Jan. 8-10, 2024
Coagulant Therapeutics to Present at Biotech Showcase 2024™
BERKELEY, Calif., Jan. 8-10, 2024
Coagulant Therapeutics today announced that Terry Hermiston, Ph.D., CEO and Founder, will be presenting Coagulant Therapeutics at the Biotech Showcase™ 2024 conference.
Coagulant Therapeutics, leaders in the development of treatments for acute bleeding, is developing a product candidate licensed from Bayer AG for the treatment of acute bleeding, CT-001, for severe postpartum hemorrhage (sPPH). In addition, Coagulant has generated a pipeline of additional clinical leads from its proprietary nanobody library to activated protein C (APC) to treat acute bleeding and sepsis.
“We started the company in 2019, and over the past two years, we have moved ever closer to making the rapid and effective treatment of acute bleeds a reality. The superior activity of CT-001 has been recently validated in clinically relevant postpartum blood and the data are now published (Sim DS et al CT-001, a novel fast-clearing Factor VIIa, enhanced the hemostatic activity in postpartum samples. Blood Adv 2024, 8:287). We are actively advancing the molecule towards clinical development. My team and I are excited about the potential of ultimately delivering a product that could have such a profoundly positive impact on so many patients and their families”, said Dr. Terry Hermiston, CEO and Founder of Coagulant . “We are further developing this space with our antibody approaches to APC where we have identified lead candidates for the treatment of trauma-induced coagulopathy and sepsis. We are excited in seeing all of this exciting science translating in medicines.”
Biotech Showcase, produced by Demy-Colton and EBD Group, is an investor conference focused on driving advances in therapeutic development by providing a sophisticated networking platform for executives and investors that fosters investment and partnership opportunities. The conference takes place each year during the course of one of the industry's largest gatherings and busiest weeks.
Nov. 15, 2023
Coagulant Therapeutics Announces Publication in Blood Advances Describing Superior Activity of CT-001 versus rFVIIa in Peripartum Patient Blood
Study Conducted at Duke University and Pusan National University Hospital, Korea Demonstrates Enhanced Activity of CT-001 relative to rFVlla in Peripartum Patient Blood
BERKELEY, Calif., Nov. 15, 2023- Coagulant Therapeutics Corporation, a privately held company focused on the design, development and commercialization of therapeutics targeted to the coagulation cascade and its adjacencies, announced the online publication of a manuscript titled " CT-001, a novel fast-clearing Factor VIIa, enhanced the hemostatic activity in postpartum samples " in the journal Blood Advances, a journal of the American Society of Hematology.
Severe postpartum hemorrhage (sPPH) unresponsive to first- and second-line therapies is treated by massive transfusion and aggressive invasive procedures to control blood loss. While rFVIIa has demonstrated activity in this setting, this has been reported to come at an increased risk of thromboembolism. CT-001 was engineered for enhanced activity and rapid clearance and this has been previously demonstrated relative to rFVIIa in both the in vitro and in vivo settings. This study extends these observations and outcomes to ex vivo peripartum blood samples.
"Death from postpartum hemorrhage occurs when the usual causes (uterine or surgical) spiral out of control, coagulation fails and exsanguination or organ failure follow. Our use of CT-001 ex vivo has shown that it has the potential to prevent or reverse coagulation failure, and, therefore, prevent exsanguination, organ failure and death from postpartum hemorrhage.", said Dr. Andra James, MD, Professor Emeritus of Obstetrics and Gynecology at Duke University, senior author of the study.
“sPPH is the leading cause of maternal morbidity and mortality worldwide. This study further demonstrates the enhanced activity of our novel rFVIIa molecule, CT-001, relative to rFVIIa, now in peripartum blood samples and supports its development as a treatment of sPPH,” said Dr. Hermiston.
Coagulant also announces that they have been accepted into the Advanced Therapies Stream of the Creative Destruction Lab (CDL) program. CDL is a nonprofit organization that delivers an objectives-based program for massively scalable, seed-stage, science- and technology-based companies. Coagulant will have the opportunities to work with top mentors in the industry to bring forward a pipeline of assets into clinics.
Aug. 1, 2023
Coagulant Therapeutics Announces Publication in Journal of Trauma and Acute Care Surgery Describing CT-001 Activity in the Setting of Coagulopathy
Manuscript Characterizes Superior In Vivo Activity of CT-001 relative to rFVIIa under Coagulopathic Conditions
BERKELEY, Calif., Aug. 1, 2023 – Coagulant Therapeutics Corporation, a privately held company focused on the design, development and commercialization of therapeutics targeted to the coagulation cascade and its adjacencies, announced the online publication of a manuscript titled “Rapid Clearing CT-001 Restored Hemostasis in Mice with Coagulopathy Induced Activated Protein C” in the Journal of Trauma and Acute Care Surgery[1].
CT-001 is a next-generation recombinant Factor VIIa (rFVIIa)-based therapy, engineered to limit its circulating half-life to approximately three (3) minutes in comparison to a half-life of about two (2) hours for wild type (WT) rFVIIa in mice. This acceleration in clearance time from the body leads to improved safety in thrombogenicity models. In addition, CT-001 has been designed to have enhanced affinity for phosphatidylserine on the cell surface which leads to an increase in activity. These modifications result in superior efficacy and safety of CT-001 over rFVIIa both in vitro and in animal models.
“Uncontrolled bleeding is the largest cause of death among individuals’ ages 1 to 44 years,”[2] said Terry Hermiston, Ph.D., founder and CEO of Coagulant Therapeutics. “Coagulopathy induced by trauma results in more severe bleeding, multi-organ failure and higher mortality. Hence it is important that any molecule being developed to treat acute bleeding demonstrate activity in this setting – and CT-001 has.”
Characterization and in vivo validation of CT-001 in an APC-induced model of coagulopathy
The in vivo studies compared the safety and efficacy of CT-001 versus recombinant FVIIa in an activated protein C (APC) induced model of coagulopathy. In this model, APC induces coagulopathy through the breakdown of coagulation factors V and VIII, compromising the activity of the normal coagulation cascade seen in patients with acute traumatic coagulopathy. These studies confirmed that the short acting CT-001 is active in vivo in this model of coagulopathy and superior to rFVIIa, further demonstrating the enhanced activity of CT-001 versus rFVIIa in the in vivo setting.
“These studies support the potential utility of CT-001 in acute bleeding settings where there currently is no approved pharmaceutical therapies. We are preparing for a Phase 1 clinical trial in the initial indication of severe postpartum hemorrhage, an indication of significant unmet medical need, where we were recently granted orphan drug designation” stated Dr. Hermiston.
[1] Sim DS, Mallari CR, Bauzon M, Hermiston TW. Rapid Clearing CT-001 Restored Hemostasis in Mice with Coagulopathy Induced by Activated Protein C. J Trauma Acute Care Surg. 2023 Jun 19. doi: 10.1097/TA.0000000000004079. Epub ahead of print. PMID: 37335129.
[2] Centers for Disease Control and Prevention, National Center for Injury Prevention and Control
Jun. 27, 2023
Coagulant Therapeutics Presents Data on an additional APC antibody library and on the activity of CT-001 in peripartum patient samples at the the 2023 31st Congress of the International Society on Thrombosis and Haemostasis Annual Meeting
Coagulant Therapeutics Presents Data on an additional APC antibody library and on the activity of CT-001 in peripartum patient samples at the the 2023 31st Congress of the International Society on Thrombosis and Haemostasis Annual Meeting
Poster presentations describe a novel antibody-engineered library against APC and the superior activity of CT-001 versus rFVIIa in peripartum patient blood\
MONTREAL, Jun. 27, 2023 – Coagulant Therapeutics Corporation, a privately held company focused on the design, development and commercialization of therapeutics targeted to the coagulation cascade and its adjacencies, announced data from an additional antibody library to activated protein C (APC) in a poster titled “Single Amino Acid Substitutions of an Antibody Specific for a Non-Active Site of Activated Protein C (APC) Yielded an Antibody Library Capable of Modulating and Uncoupling APC Pleiotropic Functions” and on further studies in peripartum patient samples in a poster presentation titled “CT-001, A Novel Factor VIIa, Demonstrates Enhanced In Vitro Procoagulant Activity in Peripartum Patient Samples” at the 31st Congress of the International Society on Thrombosis and Haemostasis (ISTH) Annual Meeting in Montreal, Quebec, June 24-28, 2023.
APC has a role in regulating coagulation, inflammation, and cell survival, and thus is important for maintaining hemostatic balance during health and disease. In the new antibody library presented as a poster at ISTH, single amino acid mutations were generated in an antibody previously identified to a single epitope of APC to understand how single amino acid substitutions might affect and differentiate the well described pleiotropic properties of the protein. Developing APC-based therapeutics has been a goal for therapeutics for decades but the ability to selectively eliminate or retain these functions individually, or in combination, has been challenging. Importantly, this gives Coagulant two highly differentiated libraries to query and generate additional candidates for APC-targeted therapeutic interventions.
“In our APC libraries we believe that we have the most extensive collection of APC directed antibodies for the screening and identification of candidate antibodies for the treatment of an array of diseases, including trauma. We are very excited to explore their potential” said Dr. Terry Hermiston, Ph.D., CEO of Coagulant Therapeutics.
In the second poster at ISTH, scientists at Coagulant Therapeutics along with clinicians from Duke University, in Durham, North Carolina, USA, and Pusan National University School of Medicine, Busan, Republic of Korea led by Drs. Andra James and Seung-Chul Kim,
respectively, describe how in the evolving hemostatic landscape in the pre- and post-delivery patient samples, CT-001 is able to maintain superior activity to recombinant FVIIa (rFVIIa). Severe postpartum hemorrhage (sPPH) unresponsive to first- and second-line therapies is treated by massive transfusion and aggressive invasive procedures to control blood loss. While rFVIIa has demonstrated activity in this setting, this has been reported to come at an increased risk of thromboembolism. CT-001, was engineered for enhanced activity and rapid clearance and this has been previously demonstrated relative to rFVIIa in both the in vitro and in vivo settings. This study extends these observations and outcomes to ex vivo peripartum blood samples.
“Severe postpartum hemorrhage is the leading cause of maternal death worldwide. These studies in peripartum blood samples further support the enhanced activity of our novel rFVIIa molecule, CT-001, relative to rFVIIa, and together with our previous studies, support its advancement to the clinic for the treatment of sPPH,” said Dr. Hermiston.
Mar. 21, 2023
Tenant Spotlight on Coagulant, a Company Defying the Odds to Save Lives
From Baker Labs, powered by QB3
Whether it’s because of car accidents or childbirth, sudden and severe bleeding is the most common cause of death for young people. So why are there no FDA-approved treatments for it?
“Why? Simple – most diseases are diagnosed, like cancer or cardiovascular disease, and then treated during a scheduled appointment. Acute bleeding is different. It occurs unpredictably and acutely. You must get to a hospital and hope that you are in time for a life-saving procedure,” says Terry Hermiston, founder and CEO of Coagulant Therapeutics.
When emergencies arise, doctors often have no other choice but to use drugs that are not approved to treat acute bleeding. One such drug is “recombinant Factor VIIa” (rFVIIa). While it may help stop bleeding, it remains active in the body for several hours, increasing the risk of fatal blood clots. But what if that could be fixed?
That is the goal of Terry and Coagulant CSO Derek Sim. The two began to develop the idea for a potential treatment when they launched the acute bleeding program at Bayer’s now-closed San Francisco Open Innovation Center. Terry left in 2017 to pursue entrepreneurial projects. Along with CMO Dr. Frank Booth, Terry and Derek are working to transform the current treatment landscape in acute bleeding. If they’re successful, their therapy could save up to 40% of the lives that would otherwise have been lost.
“We had a simple hypothesis – shorten the time of the activated coagulation factor in circulation, to reduce the possibility of unwanted blood clots, to increase its safety,” Terry explains. “To that end, we engineered rFVIIa to be fast-clearing, reducing its circulating half-life from 2 hours to 3 minutes.” Pre-clinical studies of their drug, CT-001, demonstrate that it rapidly clears out, reducing the risks of unwanted blood clots without compromising activity and efficacy.
Developing a treatment for acute bleeding is a tremendous opportunity to save lives, but it is also a unique market— a potentially multibillion dollar one at that. Because Coagulant is not “sharing” a piece of the market, as they would in oncology or cardiovascular disease, the market is theirs to build and develop. But as with every worthy cause, they face significant challenges.
One such challenge is how to conduct clinical trials. Because acute bleeding is unpredictable and varies in severity, more patients are required for clinical studies, which makes development more expensive. This is a barrier to Coagulant in the absence of significant investors and pharma partners. To circumvent this challenge, they sought to prove the efficacy of their treatment in a more cost-effective fashion by targeting severe postpartum hemorrhage.
“This condition is a leading cause of mortality in women during childbirth,” Terry says. “Approximately 5% of women continue to bleed following childbirth. They are then treated with uterotonics – treatments to contract the uterus – and this is often successful. However, if this fails, the mother is then subjected to an invasive procedure – embolization, ligation, or hysterectomy – costly procedures that can end in loss of fertility or even cost the life of the mother.”
Their preclinical work has now been published on CT-001, and they have received orphan drug designation for severe postpartum hemorrhage. But it doesn’t end there. Coagulant is building a pipeline of other molecules for treating acute bleeding in trauma and hemophilia. Recently, they presented at the American Society for Hematology on their unique nanobody library and shared that they have found leads to potential treatments for treating trauma and hemophilia with further potential expansion into adjacencies such as sepsis. Their manuscript on this was recently published in Blood Advances, a journal of the American Society of Hematology.
“I think anyone who meets me understands my passion for what I’m doing,” Terry says. “I’m not here to make a business per se. I am here to impact people’s lives. Sure, I hope it becomes a business in the process, but as I told my team, we’re going to be the first ones to solve this problem. And if nothing else, I think we can look back at ourselves at the end of this journey and know that we’re creating a movement in this field.”
Feb. 27, 2023
Coagulant Therapeutics Announces Publication in Blood Advances Describing Novel Nanobody Library to Activated Protein C (APC) and Initial Leads for Treatment of Trauma and Hemophilia
Manuscript Characterizes Novel llama-based Antibody Library to APC as Source of an Array of Disease Treatments
BERKELEY, Calif., Feb. 27, 2023 /PRNewswire/ -- Coagulant Therapeutics Corporation, a privately held company focused on the design, development and commercialization of therapeutics targeted to the coagulation cascade and its adjacencies, announced the online publication of a manuscript titled "Selective Modulation of Activated Protein C Activities by a Non-Active Site Targeting Nanobody Library" in the journal Blood Advances, a Journal of the American Society of Hematology.
Activated Protect C (APC) has a role in regulating coagulation, inflammation, and cell survival, and thus is important for maintaining the homeostatic balance during health and disease. Developing APC-based therapeutics that selectively eliminate or retain these functions individually, or in combination, has been challenging. Leveraging the unique properties of llama antibodies (referred to as nanobodies) the manuscript describes the generation of a novel nanobody library to APC. The manuscript goes on to demonstrate that by using assays specific for coagulation, inflammation and cell survival functions of APC, the investigators were able to identify an array of human APC specific antibodies able to retain or eliminate these functions. This represents a tool chest for APC targeted therapies that has already identified a lead candidate for the treatment of trauma and hemophilia.
"The ability of the Coagulant Therapeutics APC nanobody library to selectively change the activity profile of APC is quite amazing." said Dr. Laurent Mosnier, Ph.D., Professor in Department of Molecular Medicine at the Scripps Research Institute, and senior author on the publication. "Not only were we able to select for the beneficial activities of APC while eliminating unwanted activities at the same time, but we were also able to improve the cytoprotective effects of APC to enhance the therapeutic properties of this blood coagulation enzyme that is implicated to play a role in many different diseases."
The mechanism of action differs from Coagulant Therapeutics' lead molecule, CT-001, an engineered highly potent, fast clearing FVIIa molecule being developed for the treatment of severe postpartum hemorrhage, the leading cause of maternal mortality globally. As a result, these differing approaches of targeting APC with a nanobody and utilizing a modified coagulation factor may prove to be complementary or even synergistic to one another in the clinic.
"Our novel nanobody library to the human APC protein offers potential treatments for acute bleeding conditions, such as trauma as well as indications beyond acute bleeding where APC play an important role, such as hemophilia and sepsis" said Terry Hermiston, Ph.D., founder and CEO of Coagulant Therapeutics. "APC associated diseases like trauma and sepsis are areas of significant unmet medical need and we view our library and lead molecules as exciting opportunities to explore novel therapies in this space."
Feb. 13, 2023
Coagulant Therapeutics Presents Data on CT-001 activity in peripartum patient samples at the 2023 Society for Maternal-Fetal Medicine 43rd Annual Pregnancy Meeting
Poster presentation describes enhanced activity of CT-001 in peripartum patient blood, supporting advancement as a treatment to severe postpartum hemorrhage
SAN FRANCISCO, Feb. 13, 2023 /PRNewswire/ -- Coagulant Therapeutics Corporation, a privately held company focused on the design, development and commercialization of therapeutics targeted to the coagulation cascade and its adjacencies, announced data from pre-clinical studies on CT-001, an FVIIa derivative engineered for superior safety and efficacy in a poster presentation titled "CT-001, A Novel Next Generation Factor VIIa, Demonstrates Enhanced Procoagulant Activity in Peripartum Patient Samples" at the Society For Maternal-Fetal Medicine 43rd Annual Pregnancy Meeting in San Francisco, California, February 6-11, 2023.
Severe postpartum hemorrhage (PPH) unresponsive to first- and second-line therapies is treated with massive transfusion and aggressive procedures to control blood loss. Recombinant factor VIIa (rFVIIa) has demonstrated some efficacy but an increased risk of thromboembolism has been reported. To address this liability, a novel rFVIIa, CT-001, engineered for enhanced activity and rapid clearance was developed. In previous pre-clinical studies, CT-001 has shown rapid clearance and low thrombogenicity. In this study, conducted at the Departments of Obstetrics and Gynecology, Duke University, Durham, NC, USA, and Pusan National University School of Medicine, Busan, Rep of Korea, respectively, CT-001 hemostatic activity was assessed ex vivo in peripartum blood samples.
"There are limited therapeutic options and few data from clinical studies to help guide the hemostatic management of severe postpartum hemorrhage, the leading cause of maternal death worldwide. Our use of CT-001 ex vivo shows promise and opens the door to future clinical studies", said Dr. Andra James, MD, Professor Emeritus of Obstetrics and Gynecology at Duke University, senior author of the study.
"These studies in peripartum blood samples further support the enhanced activity of our novel rFVIIa molecule, CT-001, supporting its advancement to the clinic for the treatment of severe postpartum hemorrhage ," said Terry Hermiston, Ph.D., founder and CEO of Coagulant Therapeutics.
Dec. 11, 2022
Coagulant Therapeutics Presents Data on an Exosite-Specific Nanobody Library to Activated Protein C (APC) at the 2022 American Society of Hematology (ASH) Annual Meeting
Oral presentation describes a unique APC nanobody library and its potential as a source for novel treatments to diseases including trauma, hemophilia, ischemia and sepsis.
SAN FRANCISCO, Dec. 11, 2022 /PRNewswire/ -- Coagulant Therapeutics Corporation, a privately held company focused on the design, development and commercialization of therapeutics targeted to the coagulation cascade and its adjacencies, announced data from pre-clinical studies on a novel llama-derived antibody (nanobody) library directed to the APC exosite. An oral presentation titled "Selective Modulation of Activated Protein C Activities by an Exosite-Specific Nanobody Library" will be presented at the American Society of Hematology Annual Meeting in New Orleans, Louisiana, December 10-13, 2022.
"This nanobody library not only offers potential treatments for acute bleeding conditions, such as trauma and hemorrhagic stroke, it also provides the opportunity to identify possible treatments in indications beyond acute bleeding where APC plays an important role, such as hemophilia and sepsis ," said Terry Hermiston, Ph.D., founder and CEO of Coagulant Therapeutics. Dr. Hermiston pointed out that the different mechanisms of action between the APC-targeting molecules and the companies lead molecule, CT-001, an engineered highly potent, fast clearing FVIIa molecule, suggest that these molecules may actually complement one another in the clinic. "We are excited to explore their potential as additive or synergistic agents in the treatment of acute bleeds."
The data, presented by Derek Sim, Ph.D., Chief Scientific Officer for Coagulant Therapeutics, described novel llama-derived nanobodies to the exosite of Activated Protein C (APC) that affected anticoagulation, histone cleavage and receptor mediated cleavage activities of APC. The characterization resulted in 13 novel activity profiles that can be employed to regulate specific APC functions for therapeutic purposes.
"APC differs from the parent molecule protein C (PC) by the absence of 12 amino acids. Our hope was to use the unique properties of nanobodies to generate antibodies that selectively inhibit some of the APC associated functions" reported Dr. Sim "and we are very pleased to report the number and surprising diversity of APC-specific nanobodies, exemplified by LP 11, whose activity profile of hemostasis and cytoprotection promotion make it an appealing candidate for the treatment of Hemophilia and Trauma, respectively."
In addition to the oral presentation on the APC library, Coagulant presented a poster on their lead molecule, CT-001.
Abstract Title: CT-001, a Rapid Clearing Factor VIIa, Provides Pro-Hemostatic Activity to Reduce Blood Loss in Coagulopathic Conditions Induced by Activated Protein C
Abstract Number: 2458
Session Name: 321. Coagulation and Fibrinolysis: Basic and Translational: Poster II
Session Date: Sunday, December 11, 2022
Presentation Time: 6:00-8:00 PM CST/7:00-9:00 PM EST
Presenter: Dr. Derek Sim
Summary: These data, presented by Derek Sim, Ph.D., the Chief Scientific Officer of Coagulant Therapeutics, suggest that a FVIIa molecule engineered to be both safer and more active than recombinant FVIIa (rFVIIa) can be an effective treatment for acute bleeding, including bleeding with Coagulopathy, broadly defined as a derangement of hemostasis resulting in impaired clot formation. CT-001 is being developed as a new treatment for severe postpartum hemorrhage, a growing problem and a significant factor in maternal morbidity and mortality not only in the US but globally. These studies support its potential broader use in coagulopathic settings such as trauma.
About CT-001
CT-001 is an engineered version of FVIIa that is designed to address the limitations of the recombinant FVIIa (rFVIIa), approved for use in Hemophilia A and B patients with inhibitors, congenital FVII deficiency, and Glanzmann’s thrombasthenia with refractoriness to platelet transfusions but not approved for use in the acute bleeding settings such as trauma, traumatic brain injury, or intracranial hemorrhage. To improve safety, and to address the unwanted clotting risk with rFVIIa, CT-001 was engineered for rapid clearance from the blood. This feature reduces the time in which an individual is exposed to pro-coagulant activity, which reduces the risk of thromboembolic events. To improve efficacy, while compensating for the rapid removal of CT-001 from the blood, the molecule has also been engineered to target the site of bleeding more efficiently and effectively than rFVIIa, with resultant increase in activity. CT-001 was originally developed by Dr. Hermiston while he served as Vice President of Biologics at Bayer AG and subsequently acquired by the Company. CT-001 is currently under investigation for treatment of severe postpartum hemorrhage.
About APC Antibody Program
APC is a pleiotropic enzyme that has been identified as a target in the treatment of a variety of disease indications including Trauma, Hemophilia, Ischemia and Sepsis. Coagulant Therapeutics Corporation has initiated a research program into the identification of APC-directed therapeutic antibodies, leveraging the unique features of llama antibodies (nanobodies) to derive novel nanobodies to the exosite of the protein. The nanobody library has an array of different antibodies to differing epitopes and, importantly, generated a lead which is being studied for treatment of trauma and hemophilia, respectively. As these nanobodies have a different target and mechanism of action to CT-001, it is anticipated that these can be independent treatments with the potential for additive or synergistic effects to CT-001 in the acute bleeding setting.
Media Contact:
Eliza Schleifstein
917-763-8106
Nov. 16, 2022
Coagulant Therapeutics Announces Upcoming Oral and Poster Presentations at the 2022 American Society of Hematology (ASH) Annual Meeting
SAN FRANCISCO, Calif. – (PRNEWSWIRE) -- NOV. 16, 2022 – Coagulant Therapeutics Corporation, a privately held company focused on the design, development and commercialization of therapeutics targeted to the coagulation cascade and its adjacencies, today announced the acceptance of two abstracts for presentation at the 64th American Society of Hematology (ASH) Annual Meeting, taking place in New Orleans, Louisiana and virtually December 10-13, 2022. Abstract 402 is an oral presentation highlighting Coagulant’s unique nanobody library targeting activated protein C (APC) for acute bleeding (e.g. trauma) and adjacencies (e.g. Hemophilia, Ischemia, Sepsis). Abstract 2458 is a poster describing CT-001 activity under coagulopathic conditions, a shared feature of the most fatal acute bleeds.
The accepted abstracts are listed below and available on the ASH conference website:
https://www.hematology.org/meetings/annual-meeting/abstracts
Oral Presentation
Abstract Title: Selective Modulation of Activated Protein C Activities by an Exosite-Specific Nanobody Library
Abstract Number: 402
Session Name: 321. Coagulation and Fibrinolysis: Basic and Translational
Session Date: Sunday, December 11, 2022
Presentation Time: 10:45-11:00 AM CST/11:45-12:00 PM EST
Presenter: Derek Sim, Ph.D.
Poster Presentation
Abstract Title: CT-001, a Rapid Clearing Factor VIIa, Provides Pro-Hemostatic Activity to Reduce Blood Loss in Coagulopathic Conditions Induced by Activated Protein C
Abstract Number: 2458
Session Name: 321. Coagulation and Fibrinolysis: Basic and Translational: Poster II
Session Date: Sunday, December 11, 2022
Presentation Time: 6:00-8:00 PM CST/7:00-9:00 PM EST
Presenter: Derek Sim, Ph.D.
About CT-001
CT-001 is an engineered version of FVIIa that is designed to address the limitations of the recombinant FVIIa (rFVIIa), approved for use in Hemophilia A and B patients with inhibitors, congenital FVII deficiency, and Glanzmann’s thrombasthenia with refractoriness to platelet transfusions but not approved for use in the acute bleeding settings such as trauma, traumatic brain injury, or intracranial hemorrhage. To improve safety, and to address the unwanted clotting risk with rFVIIa, CT-001 was engineered for rapid clearance from the blood. This feature reduces the time in which an individual is exposed to pro-coagulant activity, which reduces the risk of thromboembolic events. To improve efficacy, while compensating for the rapid removal of CT-001 from the blood, the molecule has also been engineered to target the site of bleeding more efficiently and effectively than rFVIIa, with resultant increase in activity. CT-001 was originally developed by Dr. Hermiston while he served as Vice President of Biologics at Bayer AG and subsequently acquired by the Company. CT-001 is currently under investigation for treatment of severe postpartum hemorrhage.
About APC Antibody Program
APC is a pleiotropic enzyme that has been identified as a target in the treatment of a variety of disease indications including Trauma, Hemophilia, Ischemia and Sepsis. Coagulant Therapeutics Corporation has initiated a research program into the identification of APC-directed therapeutic antibodies, leveraging the unique features of llama antibodies (nanobodies) to derive novel nanobodies to the exosite of the protein. The nanobody library has an array of different antibodies to differing epitopes and, importantly, generated a lead which is being studied for treatment of trauma and hemophilia, respectively. As these nanobodies have a different target and mechanism of action to CT-001, it is anticipated that these can be independent treatments with the potential for additive or synergistic effects to CT-001 in the acute bleeding setting.
Jul. 12, 2022
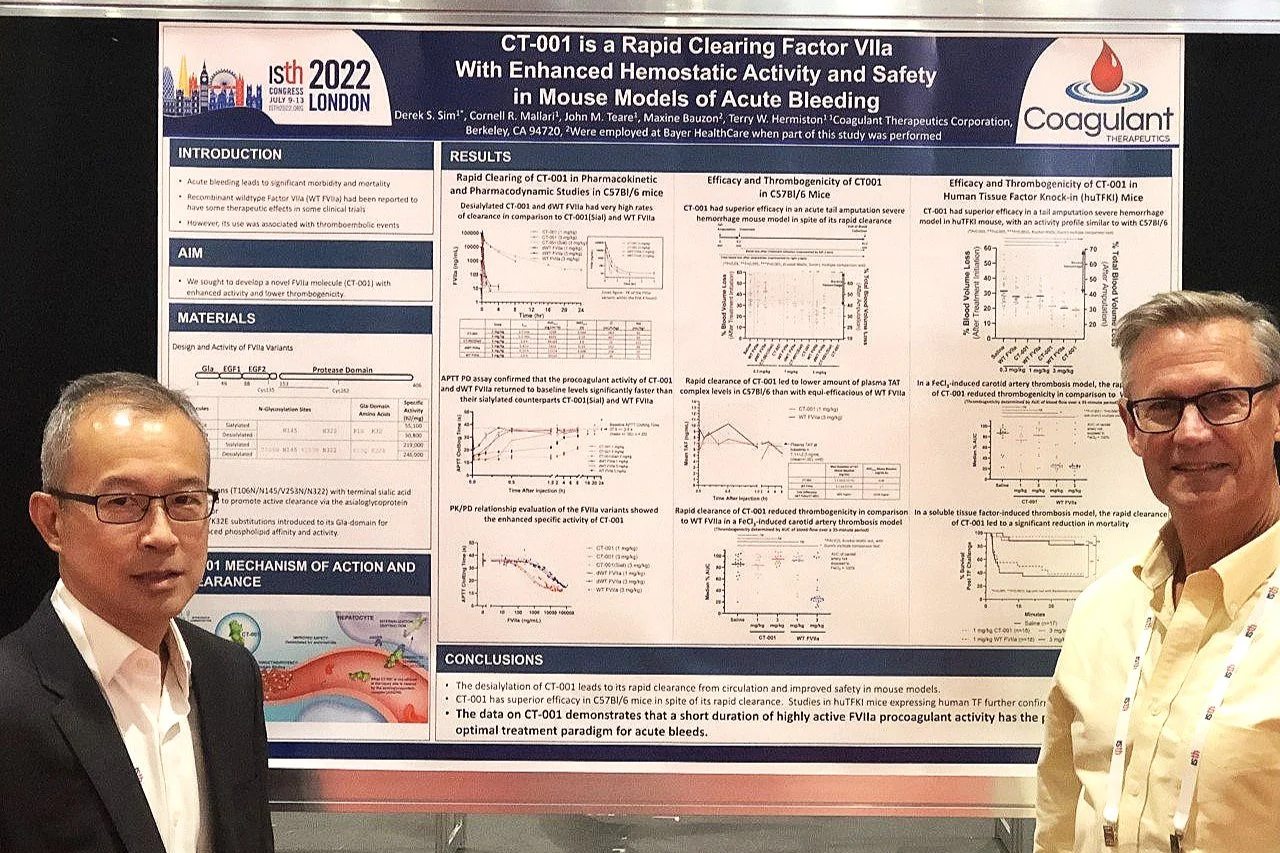
Coagulant Therapeutics Presents Data for Severe Postpartum Hemorrhage Clinical Candidate, CT-001, at International Society for Thrombosis and Haemostasis Congress
Cision distribution by PR Newswire
In vivo data suggests that CT-001, a short duration of highly active FVIIa procoagulant, has the potential to be an optimal treatment for acute bleeds.
CT-001 has also received orphan-drug designation for treatment for postpartum hemorrhage
SAN FRANCISCO, July 12, 2022 /PRNewswire/ -- Coagulant Therapeutics Corporation, a privately held company focused on the research, development and commercialization of therapeutics targeted to the coagulation cascade and its adjacencies, announced data from in vivo pre-clinical studies of its lead investigational candidate CT-001. CT-001 is the only engineered FVIIa therapy under development to treat postpartum hemorrhage. These data were shared during a poster presentation (abstract number PB1003; July 12 Tuesday, 2022) at the International Society on Thrombosis and Haemostasis (ISTH) in London, July 9-13, 2022.
Dr. Derek Sim, Chief Scientific Officer of Coagulant Therapeutics, suggests that these data demonstrate that a FVIIa molecule engineered to be both safer and more active than recombinant FVIIa (rFVIIa) may be an effective treatment for acute bleeding.
Gla (gamma-carboxyglutamic acid) domain engineering increases the activity of CT-001 2-5 fold over rFVIIa. To reduce unwanted thromboembolic events that have been described for the rFVIIa molecule, Coagulant also applied desialylation to the molecule. This process exposes galactose residues on the molecule for recognition and active clearance by the asialoglycoprotein receptor, which reduces the circulation time in the body from 2 hours down to 3 minutes. This reduced circulation time resulted in significant reduction in thrombogenicity risk in 2 mouse models. Importantly, the combination of superior activity and shorter circulation time of CT-001 translated into superior efficacy in mouse models of acute bleeding.
"We are excited to present our data at ISTH and to further engage the scientific and clinical communities around our molecule and its application to acute bleeding," said Terry Hermiston, Ph.D., founder and CEO of Coagulant Therapeutics. "Our goal is to advance CT-001 to the clinic as a new treatment for severe postpartum hemorrhage, a growing problem and a significant factor in maternal morbidity and mortality not only in the US but globally."
The Company also announced that the U.S. Food and Drug Administration (FDA) has granted CT-001orphan drug status as a human recombinant factor VIIa variant, desialylated for the treatment of postpartum hemorrhage.
About CT-001
CT-001 is an engineered version of clotting factor FVIIa that is designed to address the safety and efficacy limitations of the recombinant FVIIa(rFVIIa). Recombinant FVIIa is currently approved for use in Hemophilia A and B patients with inhibitors, congenital FVII deficiency, and Glanzmann's thrombasthenia with refractoriness to platelet transfusions, however rFVIIa is not currently approved for use in acute bleeding settings such as trauma, traumatic brain injury, intracranial hemorrhage or severe postpartum hemorrhage. To improve safety and address the unwanted clotting risk associated with rFVIIa, CT-001 was engineered for rapid clearance from the blood. This feature reduces the time during which an individual is exposed to pro-coagulant activity, and therefore reduces the risk of thromboembolic events. To improve efficacy, while compensating for the rapid removal of CT-001 from the blood, the molecule has also been engineered to target the site of bleeding more efficiently and effectively than rFVIIa. This targeting results in increased activity. CT-001 was originally developed by Dr. Hermiston during his tenure as Vice President of Biologics at Bayer AG and was subsequently acquired from Bayer by the Company. CT-001 is currently under investigation for treatment of severe postpartum hemorrhage.
Media Contact:
Eliza Schleifstein
917-763-8106
eliza@schleifsteinpr.com
SOURCE: https://www.prnewswire.com/news-releases/coagulant-therapeutics-presents-data-for-severe-postpartum-hemorrhage-clinical-candidate-ct-001-at-international-society-for-thrombosis-and-haemostasis-congress-301584157.html?tc=eml_cleartime
Jul. 7, 2022
Coagulant Therapeutics Announces Publication of In Vivo Data Demonstrating Safety and Efficacy of CT-001 for the Management of Acute Bleeding
Cision distribution by PR Newswire
The short duration of enhanced FVIIa procoagulant activity provided by CT-001 has the potential to be an optimal paradigm for the treatment of acute bleeds
CT-001 to be evaluated as a treatment for severe postpartum hemorrhage
SAN FRANCISCO, Jul. 7, 2022 /PRNewswire/ -- Coagulant Therapeutics Corporation, a privately held company focused on the design, development and commercialization of therapeutics targeted to the coagulation cascade and its adjacencies, today announced results from in vivo studies for its lead product candidate, CT-001, published in the July 2022 edition of the journal, Thrombosis Research.
The manuscript, entitled "CT-001 Is a Rapid Clearing Factor VIIa with Enhanced Clearance and Hemostatic Activity for the Treatment of Acute Bleeding in Non-Hemophilia Settings," showed that CT-001 is a safe and effective treatment of acute bleeding. CT-001 is a next-generation Factor VIIa (FVIIa)-based therapy, a recombinant and activated version of the coagulation factor, Factor VII, that is naturally found in the body and plays a key role in the arrest of bleeding. CT-001 has been engineered to limit its circulating half-life to only three (3) minutes in comparison to a half-life of two (2) hours for wild type (WT) FVIIa. This acceleration in clearance time from the body leads to improved safety in thrombogenicity models. In addition, CT-001 has been designed to have enhanced affinity to the cell surface which leads to an increase in activity. This results in superior efficacy in an acute bleeding model over recombinant FVIIa.
"Uncontrolled bleeding is the single largest cause of death among individuals ages 1 to 44 years. CT-001 has the potential to become the new standard of care to treat acute bleeding,"1 said Terry Hermiston, Ph.D., founder and CEO of Coagulant Therapeutics. "We believe up to 40 percent of hemorrhage-related deaths could be prevented with the type of rapid hemostatic control demonstrated by CT-001."2,3
Engineering an improved FVII molecule: Characterization and in vivo validation
The in vivo studies compared the safety and efficacy of CT-001 versus recombinant FVIIa in multiple mouse models. The studies confirmed that desialylation of the molecule increased the speed by which it cleared from the body. The resultant shortened half-life translated to reduced pathologic thrombogenicity by comparison with the conventional recombinant FVIIa in two thrombosis models. These studies also tested the efficacy of the CT-001 molecule, engineered in the Gla domain (gamma-carboxyglutamic acid, a FVII amino acid sequence responsible for binding to the site of injury) for enhanced targeting and potency to compensate for the rapid clearance. In a mouse model of acute bleeding, CT-001 prevented mice from progressing to severe hemorrhage even with the increased clearance and demonstrated superiority to the native FVIIa molecule. The in vivo studies also confirmed the results of a 2021 study of CT-001 in in vitro systems published in the journal Research and Practice in Thrombosis and Haemostasis, which demonstrated the rapid clearance and enhanced potency of CT-001 versus recombinant FVIIa.[4]
"We feel that these studies support the potential utility of CT-001 in acute bleeding settings where there currently exists no approved pharmaceutical therapies. We are preparing for a Phase 1 clinical trial in the initial indication of severe postpartum hemorrhage, an indication of significant unmet medical need where we were recently granted orphan drug designation" stated Hermiston.
About CT-001
CT-001 is an engineered version of FVIIa that is designed to address the limitations of the recombinant FVIIa, approved for use in Hemophilia A and B patients with inhibitors, congenital Factor VII (FVII) deficiency, and Glanzmann's thrombasthenia with refractoriness to platelet transfusions but not approved for use in the acute bleeding settings such as trauma, traumatic brain injury, intracranial hemorrhage or severe postpartum hemorrhage. To improve safety, and to address the unwanted clotting risk with FVIIa, CT-001 was engineered for rapid clearance from the blood. This feature reduces the time in which an individual is exposed to pro-coagulant activity, which reduces the risk of thromboembolic events. To improve efficacy, while compensating for the rapid removal of CT-001 from the blood, the molecule has also been engineered to target the site of bleeding more efficiently and effectively than conventional FVIIa, with resultant increase in activity. CT-001 was originally developed by Dr. Hermiston while he served as Vice President of Biologics at Bayer AG and subsequently acquired by the Company. CT-001 is currently under investigation for treatment of severe postpartum hemorrhage.
[1] Centers for Disease Control and Prevention, National Center for Injury Prevention and Control
[2] Tisherman SA et al., Detailed description of all deaths in both the shock and traumatic brain injury hypertonic saline trials of the resuscitation outcomes consortium.Ann Surg 2015 261:586-590.
[3] Spinella PC et al, Prehospital hemostatic resuscitation to achieve zero preventable deaths after traumatic injury. Curr Opin Hematol 2017 24:529-535.
[4] Sim DS et al., In vitro characterization of CT-001 – a short-acting factor VIIa with enhanced prohemostatic activity. Res Pract Thromb Haemost 2021 5:e12530.
Media Contact:
Eliza Schleifstein
917-763-8106
eliza@schleifsteinpr.com
SOURCE: https://www.prnewswire.com/news-releases/coagulant-therapeutics-announces-publication-of-in-vivo-data-demonstrating-safety-and-efficacy-of-ct-001-for-the-management-of-acute-bleeding-301581977.html?tc=eml_cleartime
Jan. 27, 2022
Coagulant Therapeutics Announces Key Milestones on Path to Commercialization
Developing New Treatments for Acute Bleeding
San Francisco, Calif. – Coagulant Therapeutics’ third year provides an opportunity for the company to commemorate the milestones it has reached in its attempt to develop the first approved pharmaceutical treatment for acute bleeding, the leading cause of death in humans aged 1 to 46.
“Acute bleeding exacts a terrible human toll – from the mother who hemorrhages following childbirth to the soldier bleeding from a traumatic wound sustained in action,” says Terry Hermiston, Ph.D., Coagulant Therapeutics’ founder and CEO. “And yet, despite the advances that have been made in medicine, we still lack a truly viable therapeutic treatment for acute bleeding. My team and I are excited about the potential of delivering a product that could have such a profoundly positive impact on so many patients and their families.”
Coagulant Therapeutics’ has developed a proprietary molecule, CT-001, based on a natural blood coagulation factor, FVIIa, that is approved for the treatment of uncontrolled bleeding in hemophilia patients with inhibitors, an inherited bleeding disorder. While FVIIa has been used in clinical trials to treat severe uncontrolled bleeding from other causes, the results have been mixed and raised safety concerns, particularly with regard to adverse thromboembolic events, or unwanted clotting.
“Our goal has been to learn from the first generation FVIIa drug and in doing so, we have engineered a next-generation molecule that both more potent and is rapidly cleared from the circulation, increasing the potential for targeted effective clotting at the target injury while limiting the potential for unwanted thromboembolic events. Our research has consistently found that Coagulant Therapeutics’ engineered molecule, CT-001, is highly active and efficacious with a safety profile that is superior to the native FVIIa molecule” said Hermiston.
The milestones on the path to commercialization that have been achieved by Coagulant Therapeutics since its inception in 2019 include the following:
Acquisition of acute bleeding technologies and intellectual property from Bayer HealthCare
Prior to launching Coagulant Therapeutics, Hermiston was vice president of biologics for Bayer AG in the United States. As the site head of Bayer HealthCare’s U.S. Innovation Center in San Francisco, he shared responsibility for the development of its research portfolio in hematology and, later, in immuno-oncology and antibody drug conjugates. Upon retiring, he negotiated a licensing agreement that provided him with access to Bayer FVII assets that had been explored and engineered by his team in Berkeley.
These included all FVIIa assets and intellectual property from the hemophilia drug program previously acquired by Bayer HealthCare from Redwood City, Calif.,-based Maxygen in a deal valued at $120 million. These assets have served as the platform for the development of CT-001.
“I launched Coagulant Therapeutics because I truly believed in the work we were doing at Bayer and I wanted to see it come to fruition,” Hermiston notes. “There is a significant unmet need for safe and effective treatment options for acute bleeding situations caused by severe trauma, intracranial hemorrhage, traumatic brain injury and post-partum hemorrhage. We believe our CT-001 molecule, as well as other innovations in our pipeline, can have a positive impact on the lives of these patients and their families.”
Development of a novel molecule with improved safety and efficacy
The origins of CT-001 lie with factor VII (FVII), a coagulation factor normally found in the body that plays a key role in stopping bleeding. Factor VII is a serine protease enzyme that identifies and binds to sites of tissue damage, where it is converted to factor VIIa (FVIIa), which activates blood coagulation. As noted, however, the use of native FVIIa in acute trauma has been limited by safety concerns.
In the new, engineered molecule, native FVIIa has been desialylated, which reduces the risk of clotting by enabling active and rapid clearance from the circulation. To compensate for the rapid clearance, efficacy and targeting have been enhanced through modifications to the Gla (gamma-carboxyglutamic acid) domain, an FVIIa component responsible for binding to the site of injury. These alterations make CT-001 up to three to five times more active than native FVIIa.
The results of research on CT-001’s were published in an article in the July 2021 edition of Research and Practice in Thrombosis and Haemostasis entitled “In Vitro Characterization of CT-001 – a Short-acting Factor VIIa with Enhanced Prohemostatic Activity.”
Attraction of significant investment interest
The unmet need for a drug to treat acute bleeding represents a market opportunity that could be as large as $18 billion annually. The potential market, which has no approved pharmaceutical therapies, has helped Hermiston and Coagulant Therapeutics close on an approx. $36 million Series A funding round with the support of a consortium of investors from South Korea. “We appreciate the confidence our investors have demonstrated in our vision,” Hermiston says. “Because of their enthusiastic support, we are on track to initiate our phase 1 clinical trial with CT-001 during the first half of 2023.”
Future directions
“The initiation of clinical trials is an important milestone that will transition Coagulant Therapeutics from a pre-clinical to a clinical-stage company,” Hermiston adds. “As CT-001 continues on the path toward clinical trials, we are continuing to explore additional therapeutics for various components of the coagulation cascade to further treat acute bleed patients. Our goal is to have two drugs in clinical development and a third clinical candidate identified within the next five years. This is an exciting goal that we believe is within our reach.”
About Coagulant Therapeutics Corp.
Coagulant Therapeutics Corporation (www.CoagulantTherapeutics.com) is a privately held, IND candidate stage platform and product company focused on the design, development and commercialization of therapeutics targeted to the human coagulation cascade. The lead product candidate, CT-001, is a next-generation Factor VIIa designed for enhanced efficacy and safety in the setting of acute bleeding, including severe post-partum hemorrhage (sPPH). Coagulant is also actively developing additional therapeutics for the treatment of acute bleeding and other coagulation cascade-related diseases. The Coagulant development team has deep experience in the design and development of protein therapeutics and acute bleeding product candidates, including our CEO Dr. Terry Hermiston who was previously Head of Bayer Biologics Research in the US and Site Head of the Bayer HealthCare US Innovation Center in the San Francisco Bay Area.
Jan. 10 - 12, 2022
Coagulant Therapeutics to Present at Biotech Showcase 2022™
Berkeley, CA – Coagulant Therapeutics today announced that it is presenting virtually at the Biotech Showcase™ 2022 conference with an online presentation. Registered attendees to Biotech Showcase can access Coagulant Therapeutics’ recorded company presentation twenty-four seven with on-demand access, allowing attendees to view presentations at their convenience in case scheduling does not allow viewing during the main event week.
Terry Hermiston, Ph.D., CEO and Founder, will be presenting Coagulant Therapeutics at Biotech Showcase.
Coagulant Therapeutics is developing product candidates licensed from Bayer AG for the treatment of acute bleeding, including CT-001 for severe postpartum hemorrhage (sPPH). In addition, Coagulant is developing a pipeline of internally-discovered treatments, with diverse molecular targets and mechanisms, not only in acute bleeding but extending into inherited bleeding disorders and acute care. The Coagulant team has deep understanding and experience with biologics and the coagulation cascade.
“We started the company in 2019, and over the past two years, we have moved ever closer to making the rapid and effective treatment of acute bleeds a reality. We are currently advancing development of our lead compound, CT-001, and anticipate commencing clinical trials in 1H/2023. My team and I are excited about the potential of ultimately delivering a product that could have such a profoundly positive impact on so many patients and their families”, said Dr. Terry Hermiston, CEO and Founder of Coagulant.
“We are delighted that Coagulant Therapeutics will be presenting at Biotech Showcase this year,” said Sara Demy, CEO of Demy-Colton. “Biotech Showcase is a prime occasion for life science entrepreneurs and investors to come together to discover the potential of innovative technologies that will drive the future of drug discovery.”
Biotech Showcase, produced by Demy-Colton and EBD Group, is an investor conference focused on driving advances in therapeutic development by providing a sophisticated networking platform for executives and investors that fosters investment and partnership opportunities. The conference takes place each year during the course of one of the industry's largest gatherings and busiest weeks.
ABOUT COAGULANT THERAPEUTICS
Coagulant Therapeutics Corporation (www.CoagulantTherapeutics.com) is a privately held, IND candidate stage platform and product company focused on the design, development and commercialization of therapeutics targeted to the human coagulation cascade. The lead product candidate, CT-001, is a next-generation Factor VIIa designed for enhanced efficacy and safety in the setting of acute bleeding, including severe post-partum hemorrhage (sPPH). Coagulant is also actively developing additional therapeutics for the treatment of acute bleeding and other coagulation cascade-related diseases. The Coagulant development team has deep experience in the design and development of protein therapeutics and acute bleeding product candidates, including our CEO Dr. Terry Hermiston who was previously Head of Bayer Biologics Research and Site Head of the Bayer HealthCare US Innovation Center in the San Francisco Bay Area.
About CT-001
CT-001 is a next-generation Factor VIIa product designed and engineered for the prevention and treatment of acute bleeding. Proprietary modifications were made to conventional FVIIa with the goal of targeting the molecule to sites of active bleeding, with enhanced coagulation activity and efficacy, while reducing the risk of systemic toxicities associated with conventional FVIIa. Read more here.
Coagulant Therapeutics
2630 Bancroft Way
Berkeley, CA 94704
www.coagulanttherapeutics.com
ABOUT BIOTECH SHOWCASE
Biotech Showcase is an investor and networking conference devoted to providing private and public biotechnology and life sciences companies with an opportunity to present to, and meet with, investors and pharmaceutical executives in one place. Investors and biopharmaceutical executives from around the world gather at Biotech Showcase during this bellwether week which sets the tone for the coming year. Now in its 14th year, this well-established, highly respected conference features multiple tracks of presenting companies, plenary sessions, workshops, networking, and an opportunity to schedule one-to-one meetings. Biotech Showcase is produced by Demy-Colton and EBD Group. Both organizations have a long history of producing high-quality programs that support the biotechnology and broader life sciences industry.
Follow Biotech Showcase at @EBDGroup and @Demy_Colton and tag your posts with #BiotechShowcase.